Humidity is due to the evaporation of water from the surface of the seas and oceans. Absolute humidity is the density of water vapor per unit volume, and the percentage of the amount of water vapor in a certain volume of air to the amount of vapor that can saturate this volume at a given temperature is called relative humidity . Relative humidity is subject to daily fluctuations. This is primarily due to temperature changes. The higher the air temperature, the greater the amount of water vapor required for its full saturation. At low temperatures, less water vapor is needed for maximum saturation.
During these periods, air temperature and humidity were monitored for each of the four air streams: outdoor air, supplied air, internal air, and ventilated air. The wet enthalpy of air consists of the sum of the so-called intelligent energy and latent energy. Sensitive energy refers to air temperature and latent energy to humidity, that is, to the amount of water in 1 kg of air. Pathic intelligent energy is called "heat", so enthalpy is the sum of heat and humidity.
A heat exchanger transfers heat between two air streams - the winter open cold air is heated by the evacuated internal air. An enthalpy heat exchanger transfers heat and moisture between two air streams. As a result, not only the temperature increases in winter, but also the humidity inside the intake air.
Relative humidity and saturation deficiency are important. These indicators give an idea of \u200b\u200bthe degree of saturation of air with water vapor and indicate the possibility of heat transfer through evaporation. With increasing moisture deficiency, the ability of air to receive water vapor increases. Under these conditions, heat transfer as a result of perspiration will proceed more intensively.
Figure 3 shows the effect of a heat exchanger and an enthalpy heat exchanger on the supply air humidity based on average hourly values \u200b\u200bfor eight days. The horizontal axis shows the absolute humidity of the outside air, and the absolute humidity of the intake air is shown on the vertical axis. Although heat recovery does not affect the humidity of the supply air, the recovery of enthalpy leads to a significant increase in the humidity of the intake air. This humidity is obtained from the exhaust air, that is, moisture in the house.
For humans, relative humidity 30-60% refers to the hygiene norm. Such humidity ensures the normal functioning of the body. This helps to moisturize the skin and mucous membranes of the respiratory tract and inhaled air, to some extent maintain the constancy of humidity of the internal environment of the body. Air with a relative humidity below 20% is estimated to be dry, from 71 to 85% - as moderately humid and more than 86% - as very humid. Humidity of less than 20% is accompanied by evaporation of moisture from the mucous membranes of the respiratory tract. This leads to a decrease in their filtering ability and a feeling of dry mouth. The boundary of a person’s heat balance is air temperature 40ºС and humidity 30% or air temperature 30ºС and humidity 85%.
In FIG. The 4 Moller diagrams show the hourly values \u200b\u200bof temperature and humidity for eight days with a heat exchanger and an enthalpy heat exchanger. Here, the amount of intake air is shown as follows: the outside air is shown by green dots, and the air supplied to the passenger compartment directly behind the heat exchanger is indicated by red dots. Fig. 4 shows that the temperature due to heat recovery rises to 20 ° C, which is almost to the internal temperature. Enthalpy recovery has the same effect in terms of temperature, but also increases humidity - as indicated by a black arrow pointing to the right.
Depending on the degree of humidity, the effect of temperature is felt differently. So, the high temperature of the air in combination with its low humidity is tolerated by a person much easier than with high humidity. With an increase in air humidity, an increase in body temperature, an increase in heart rate and respiration, a headache and weakness appear, a decrease in motor activity, and the loss of heat from the surface of the body by evaporation (hydration and tissue dehydration) also decreases. Saturation of air with water vapor at low temperatures will contribute to hypothermia of the body.
Figure 4 shows the yellow air exiting from the inside - it is obvious that the internal humidity is higher under the same outdoor conditions when an enthalping heat exchanger is used. During heat recovery, the relative humidity in the room during this period ranges from 35% to 45%, and the enthalpy is from 45% to 50%. From this we can conclude that the relative humidity in the room in winter due to the use of enthalpy regeneration increases to 10%.
Efficiency values \u200b\u200bfor eight days are given in table. The thermal efficiency is calculated from temperatures T, the moisture efficiency nm is calculated from the absolute humidity x according to the following formulas. For a heat exchanger, the average thermal efficiency is 89% at a moderate outdoor temperature. In cold outdoor air, condensation may occur in the return air heat exchanger, which partially blocks the return air flow. Consequently, thermal efficiency drops to 70%. Since the heat exchanger does not transfer moisture, the moisture efficiency is zero.
Condensation, condensation of water vapor is their transition to a liquid state and the formation of water droplets. Condensation occurs when air is saturated and saturated with water vapor due to its cooling. The condensation products in the atmosphere are fog and clouds. Fog is a large amount of condensation products in the surface air layers (water droplets and ice crystals). As a result of fogs, visibility deteriorates, accidents and injuries occur. It contains dust, which makes breathing difficult.
For an enthalpy heat exchanger, the average thermal efficiency of 88% was measured, and the humidity efficiency was on average 65%. These values \u200b\u200bcorrespond to the laboratory values \u200b\u200bindicated in the technical data sheets. Plant life requires certain rules from the very beginning. If the plant must have the right conditions for successful growth, it must be equipped with plenty of water, nutrients, light, carbon dioxide and, just as importantly, the right temperature. We will talk about temperature at certain stages of growth in today's article.
Source: Pharmaceutical Industry Magazine, No. 57
The first part of the article discussed aspects of interpreting the storage conditions of drugs related to temperature limits and protection from exposure to light. In this part, it is proposed to consider the issue of maintaining humidity during storage and transportation of pharmaceutical products, taking into account current regulatory requirements.
It is important to get the right temperature at the moment when we plant ourselves as seeds, germinated by seeds so that they can grow well. Sometimes it happens that compliance with these conditions is not easy if we are talking about outdoor cultivation. When we grow indoors, we are the lords of the whole situation.
Temperature at different stages of growth
Imagine elements that do not directly affect growth and climate at the plant, but thanks to the data that they give us, we help us achieve the result of what we ask. Thermometers are priced at a very reasonable level, so you should not buy them more and control different parts of the installation. This allows us to properly regulate fans, air conditioning and heating. The most suitable thermometer is one that records the maximum and minimum temperatures. Not everyone has the opportunity to constantly monitor the condition of a growing plant throughout the day, and since the temperature can fluctuate significantly day and night, this can have a terrible effect on the manufacturer. And what causes temperature fluctuations? It depends on the operation of the lamps and fans. Too low a temperature makes the plant shock and, on the contrary, too high, this leads to a weakening of the root system, and also increases the likelihood of mold. Although he does not use it in cultivation, it is important for a large number of producers. And it’s good to know that the heating element must have the same capacity as the used space of the tank. A substrate thermometer is not the most important element for monitoring temperature, but it will not damage it. Nevertheless, it is good to be able to work with the most detailed information about what conditions the plants provide. This means that the lamps must be suspended, the ventilation system is fully installed, the proven functionality of the irrigation system, the containers must be filled with a backing. This occurs at a time when the reproductive organs of the genital organs begin to appear on plants. Since most varieties bloom depending on the length of the day and night, it is necessary to go from the growth phase to the flowering phase. If all this is supported, the drying time is increased, but, on the other hand, a higher quality product is achieved.
Growing problems and solutions
If the plants do not get out of the way, this may be because the seeds are planted too deep or cause excess water or even high temperature. Destruction of clones - can lead to low humidity, low humidity in growing environments or high temperature. We can increase the humidity by opening the ventilation openings of the greenhouse and ensuring that the temperature is maintained. Therefore, it is necessary to focus on maintaining the correct humidity and temperature. Yellowing of clones - caused by poor climate or excess moisture in growing environments. When excess humidity is turned off, automatic irrigation is turned off and only when the medium is drier. In the flowering phase, the plants are dark green, and the leaves seem to drift - a possible cause is the low night temperature.- Thermometer.
- It is considered necessary to measure the temperature in the installation.
- For proper germination, seeds require darkness, moisture and heat.
- After this time, he switches to a damp cloth.
- The first visible difference will take effect in about a week.
- In selfless varieties, this is slightly different.
- And the most important thing in our article today is temperature.
- In this case, just plant new seeds.
- What will be required to resolve this problem?
- The edges of the leaves curve and fade - perhaps this is due to the poor climate.
- Damaged parts of the sheets will be deleted.
The purpose of all our articles, united under the general title “GMP / GDP Problematic Issues”, is to initiate a discussion in the professional community with the participation of experts from different countries, focusing primarily on questions of a reasonable interpretation of existing regulatory requirements and aspects of their practical application. The professional pharmaceutical community should refuse to blindly copy other people's standards, as well as tacit self-elimination of the problems generated by such copying and start publicly discussing the key problematic issues of production and distribution of medicines based on the current state of the industry and current regulatory documents.This is where we start!
Do you know why the purple variety turns purple? It can be temperature and, of course, it also affects genetic equipment. When grown under normal conditions, chlorophyll inhibits other colors, and the resulting plant color is green. In the fall cold season, chlorophyll and the purple pigment, which is in the layer just below chlorophyll, begin to turn flowers into a beautiful purple color.
Are there more children born at meteorological moments such as storms, blizzards, storms, etc. Or are they just clichés that professionals finance, don’t even care and consider themselves simply superstitions? Therefore, we asked meteorologists where this is true.
“Store in a dry place”
Perhaps the most mysterious phrase on the labeling of a drug. Labeling is intended for the consumer and should be clear to him without explanation. Most consumers quickly associate this phrase with a place where there is no dampness, no excess moisture, and "nothing is dripping from the ceiling." - So, you need to store the drug away from the bathroom and certainly not in the basement or on the balcony.
You even said before becoming pregnant that you did not know why biometeorological prediction is part of the weather forecast? And that you are not affected by the effects of the weather and its fluctuations in your psychological and physical well-being? “Beware, you shouldn't take numbers from one to three lightly during pregnancy,” says meteorologist Dagmar Hones.
Consider your personality
After all, the weather changes in the Czech Republic every four days on average, and our ancestors long ago noticed our meteor. We can find the first mention of him in the chronicles. However, the perception of how the weather affects us is individual, and its sudden changes, extreme manifestations and individual meteorological elements can affect us in different ways. For example, some expectant mothers may have high air temperatures, and when mercury rises above 30 degrees Celsius, they do not even return home.
Only the subjects of the pharmaceutical market doubt - the responsible persons of pharmacies, distributors and manufacturers of medicines. There are still no clear and unambiguous criteria for acceptability of humidity in warehouses and pharmacies, an answer to the question of the need to control humidity in refrigerators and freezers, as well as at the stages of the supply chain (during transportation) of medicines. And this despite the fact that, on the one hand, providing a given range of air humidity values \u200b\u200bhas a critical effect on the quality of the drug, and on the other hand, it can entail enormous costs associated with maintaining this range of humidity values \u200b\u200b(especially in autumn and winter).
Others have good warm weather, but cannot stay in the sun for long. Probably the biggest stress meteorological element is atmospheric pressure or its change. Perhaps the worst effect on our body is a rapid drop in air pressure, that is, when passing through the low pressure air zone or before the arrival of cold and warm bursts. As soon as the pressure drop reaches a higher value than 4 hPa in 24 hours, we may experience headaches, joints, malaise, and some mumps get worse.
Let us first dwell on the theory of the question.
Absolute and relative humidity
The ambient air always contains water in the form of individual molecules (pairs), associates of molecules (microdrops of liquid) and microcrystals (ice). Humidity is the content of water vapor in it.
The density of water vapor in the air represents it absolute humidity (g / m 3) and actually indicates the quantitative content of water vapor by weight per unit volume of air. Maximum humidity indicates the maximum amount of water vapor that can be distributed in 1 m3 of air without the formation of condensate (see Fig. 1). Provided that there is sufficient moisture in the atmosphere, the maximum (maximum) absolute humidity depends on the air temperature. The higher the temperature, the more water vapor can hold air.
Secondly, the temperature of the air, in the case of "two in one", this is especially the so-called sensory temperature. That is, how the human body perceives temperature, depending on other meteorological elements. On hot days, the sensory temperature increases due to air humidity - this is twilight. This is due to the fact that cooling by evaporation of sweat drops on the surface of the skin is more effective in dry air than in the wet state, which is no longer able to absorb water, since it is close to the state of saturation.
Fig. 1 - The maximum possible moisture content in 1 m3 of air.
In order to judge the degree of air humidity, it is important to know how close the water vapor contained in it is to its saturation point (to achieve the maximum humidity value). For this, the value relative humidity – this is the ratio of absolute air humidity or actual water vapor density (p) to maximum air humidity or saturated water vapor density (p about ) at the same temperature, expressed as a percentage.
![]()
Of key importance is the fact that the maximum possible amount of moisture in the air (of which relative humidity is considered in%) depends on the ambient temperature. It turns out, if you heat the air, then at the same absolute humidity, its relative humidity will decrease (now% is taken from a larger value). Conversely, if you cool the air, its relative humidity will increase, and that part of the water vapor that exceeds the maximum humidity for this temperature will begin to condense - fog appears, dew falls, the windows fog up. Therefore, the temperature at which water vapor becomes saturated (dew falls) is also called the “dew point”.
The elasticity (partial pressure) of water vapor in the air shows how much of the total pressure of a given volume of air column falls on the pressure of the water vapor contained in it. The elasticity of water vapor depends on the content of water vapor per unit volume of air and air temperature. It is not difficult to notice - the vapor pressure in air is the same indicator as absolute humidity, although it is expressed not through mass-volume ratios (g / m 3), but through pressure units (mbar or mm Hg).
As the main standardization value, the value of relative humidity is important to us. It approximately gives the ratio of humidity and dew point, which means it determines when it is still dry and when it is already wet. In addition, it is easier to measure.
Humidity limits
Medicines usually require storage in a dry place. Unlike the consumer, we professionals really need a common understanding of the range of acceptable values \u200b\u200bfor relative humidity. Only in most cases, professional snobbery plays a cruel joke on this issue, and, as it does not seem strange, the need to unify everything.
Consider a simple situation. See fig. 2. Take a relative humidity scale of 0-100%, and divide it into two ranges: 0-50% (dry) and 50-100% (wet, damp). That's great - if the relative humidity is less than 50%, then this is a dry place.
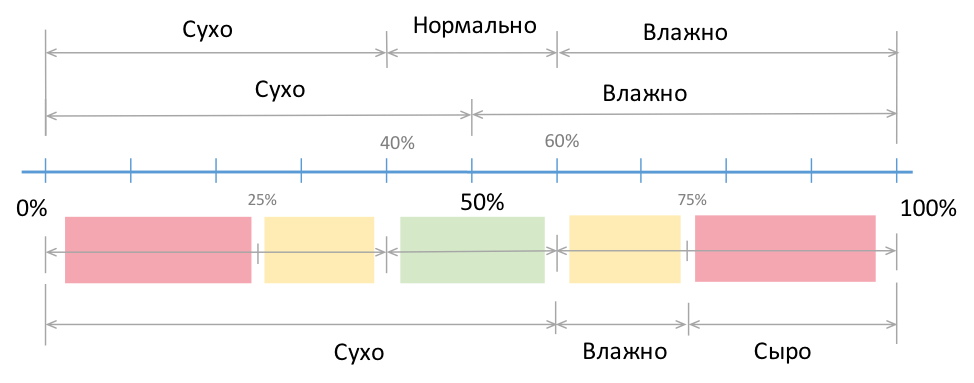
Fig. 2 Standard humidity ranges
However, often such a two-level scale is not enough to solve all the tasks assigned to business entities. Take food, for example. Is it possible to establish uniform storage conditions (for humidity, temperature) for jerky, smoked fish, chips, bread, canned goods, pasteurized milk in plastic bags, fresh vegetables, dried fruits, etc.?
Accordingly, dividing the scale into three ranges, introducing variability (50 ± 10)%, it turns out: a zone with low humidity (up to 40%), normal humidity (40-60%) and high humidity (more than 60-65%). Or, “very dry,” “dry,” “wet.” Additionally, if necessary, you can enter the fourth category - "damp" (over 75% relative humidity).
The feeling of normality of the microclimate parameters is very subjective and always based on the principle of “who has something to hurt”. Therefore, there is still no single combination of temperature and humidity, ideally suited to all existing needs, as, by the way, there is no one, single, range of storage by temperature.
Warehouses not only “live” products, people and equipment still work there. Therefore, the task is not only to create conditions for ensuring product quality, but also to preserve the life and health of service personnel, to ensure all established sanitary and hygienic standards and fire safety rules, trouble-free operation of the equipment. All this must be done taking into account the careful attitude to the ecosystem of the planet, preventing excessive, and most importantly unjustified, waste of electricity.
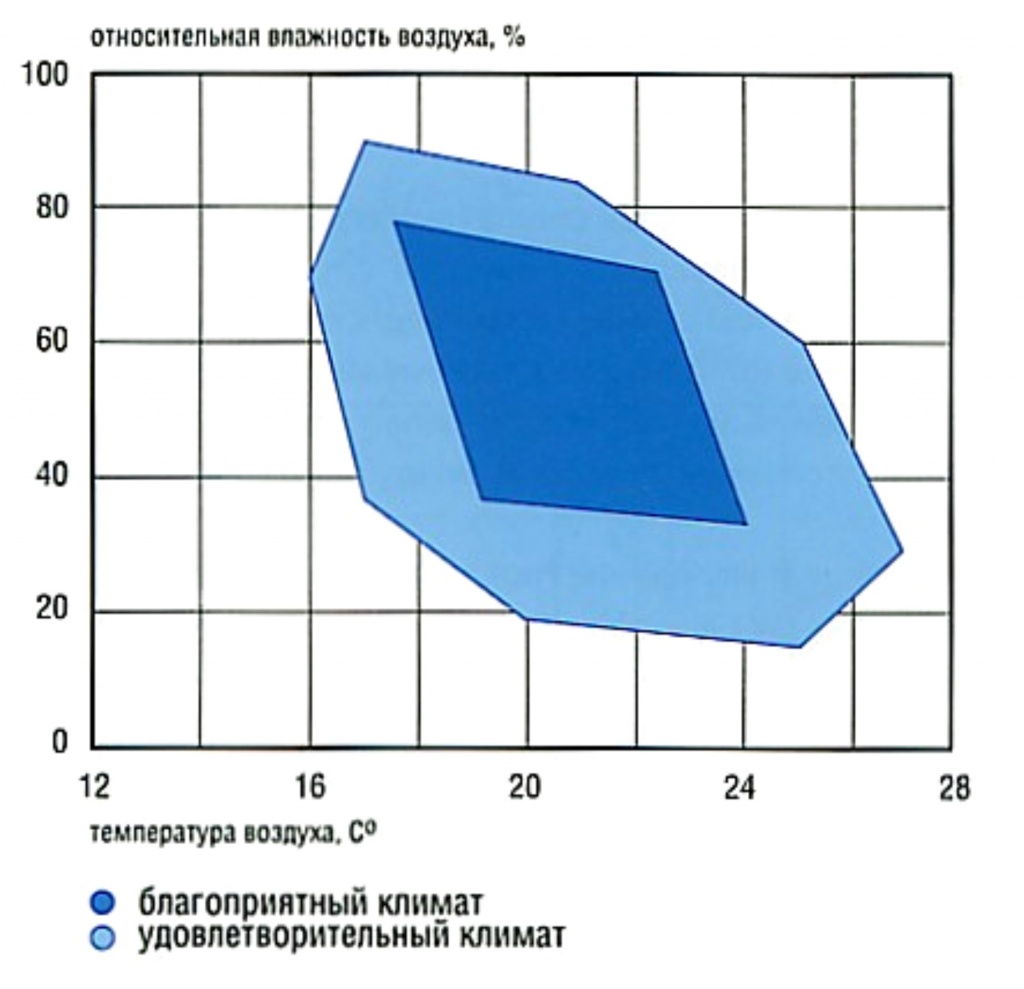
Fig. 3 - Diagram for determining a favorable climate, taking into account temperature and humidity.
Let's look at the problem with examples. First, look at Figure 3. This diagram is often given in textbooks on the design of ventilation and air conditioning systems. It establishes a comfort zone for people who can be (live, work) in a particular room. According to the diagram, the center of maximum comfort for a person is at 21 ° С and 55% humidity. Therefore, it is these numbers that are reproduced in various regulatory documents and scientific articles as ideal microclimate values. How was all this data obtained? - This is a statistical chart. To build it, several hundred healthy people were assembled and placed in a climate chamber, after which they began to change the temperature and humidity, not forgetting to interview people with a wide smile, how they feel and measure their vital signs and working capacity. What happened in the majority of the studied, then recorded. And then, in practice, repeatedly confirmed the acceptability of such results. Increased humidity (over 65-70%) causes discomfort in people (stuffy, sweating, etc.), and lowered (less than 25%) causes allergic reactions, thirst, fatigue, peeling of the skin, etc.
Now we turn to fig. 4. Here a slightly different situation is depicted. This chart has already been compiled by professionals in the field of sanitary and hygienic requirements based on data on optimal parameters for the development of adverse and aggressive environmental factors. In their opinion, the most favorable is the humidity range of 40-60%. High humidity (over 65-70%) will provoke moisture condensation on cold surfaces (for example, walls, window sills, pipelines, etc.), and most likely will lead to mold, dampening of materials, corrosion of equipment, etc., will create a comfortable environment for the propagation of microorganisms. Humidity below 15-20% under the same temperature conditions will lead to the appearance of static electricity on various surfaces, including microcircuits and boards inside computers and used electronic devices, cracking of painted and varnished surfaces, shrinkage, warping of wooden products, furniture, will contribute to the growth of bacterial flora etc.
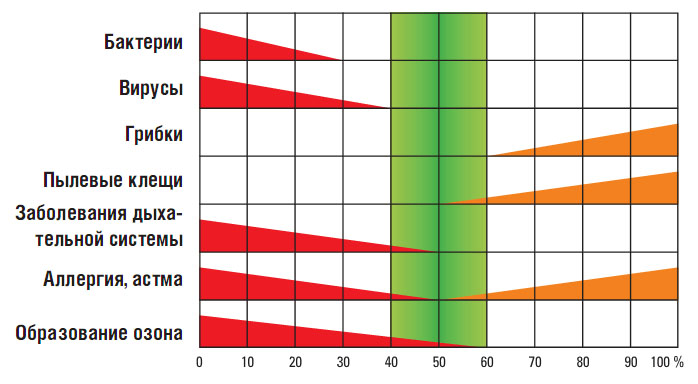
Fig. 4 Values \u200b\u200bof relative humidity influencing the development of adverse and aggressive environmental factors
Specialists responsible for the proper operation of buildings and structures have their own opinion. They will immediately indicate that in SNiPs, GOSTs and SanPiNs of the Soviet and subsequent periods figures of 30-45% relative humidity appear. But this is due only to a different climate in different regions of the USSR. For example, in Ukraine, Belarus and Moldova, you can easily maintain equilibrium relative humidity in warehouses at 40-45%, while in the Urals and Siberia, where the equilibrium humidity of street air is closer to 20%, maintaining a range of 45-50% in a warehouse is inevitable will lead to tremendous energy costs in winter.
By analogy with the food example, drug developers can also set their own requirements. Someone has crushed vegetable raw materials in filter bags, powders in a moisture-proof packaging, others have tablets coated with a moisture-proof coating, aqueous solutions in vapor-permeable containers, others have sterile preparations in ampoules, in bottles with rubber or silicone caps.
Hence the different standards. This is normal. Civil engineers write SNiPs, sanitary doctors SanPiNy, product developers - ND and specifications. And everyone is comfortable with their own range of values \u200b\u200bfor humidity. Some of its rationing is at the level of 60-65%, others - at the level of 30-35%, there are those who require to establish only a one-sided rationing interval, for example, “not higher than 50%” or “not lower than 25%”.
As a result, there was a situation when various authorized bodies cannot agree among themselves, and the business, by and large, is not interested in strict standards. It’s convenient after all. Sellers of humidifiers scare everyone with dry air, sellers of desiccants with dampness and mold. High humidity is beneficial for those who sell vapor barrier materials, any wall insulation, insulated window profiles, flowers, fresh vegetables, canned goods, etc., and frightening them with shrinkage, cracking furniture and diseases is a favorite pastime of sellers of ordinary double-glazed windows, air ionizers and vacuum cleaners for wet cleaning.
Nevertheless, summarizing regulatory documents from different industries, we can already assume the range comfortable (in all respects - for the product, and for staff, and for equipment) relative humidity at the level of 35-45% for room temperature (+20 о С), and the value of 60-65% is only its permissible upper limit.
And in this case another question is already coming to the fore. Do all medications need to be stored under the same conditions of temperature and humidity? For example, you should pay attention to the GMP requirement (part 1, paragraph 3.19): “ When designing and equipping storage areas, appropriate storage conditions should be provided. In particular, they must be clean and dry, they must be maintained at the required temperature. If required special conditions storage (e.g. temperature, humidity), it is necessary to ensure and verify such conditions, as well as to monitor them". Or to the phrase from paragraph 5.5 of the GDP rules: " Medicines must be stored separately from other products that can affect it, and defend oneselffrom the harmful effects of light, temperature, humidityand other external factors. Particular attention should be paid to products requiring special storage conditions».
Obviously, GMP / GDP's good pharmaceutical practices divide the storage conditions of drugs into ordinary (basic) and special (special) ones. and given the pharmacopoeial requirements, it turns out that everything depends on the labeling and regulatory requirements for a particular drug.
Labeling Issues
When registering medicines, national authorized bodies (expert organizations) of the countries of the former USSR persistently require a “dry” label for each drug. At the same time, international standards do not require a warning record “Keep in a dry place” on the packaging of all drugs. Especially if it is initially insensitive to moisture and (or) already packaged in containers (primary packaging) that protect the product from moisture damage.
The guidelines of the European Medicines Evaluation Agency (EMA), which establish the rules for declaring storage conditions CPMP / QWP / 609/96 / Rev2, say: “ Accurate wording must be used in the labeling of drugs entering the market. Any markings are allowed. only in cases where this cannot be avoidedas well as if it is documented that the main storage conditions are inappropriate. ” Or another instruction from the same document: “Storage conditions must be such that the consumer can comply with them. Therefore, it is necessary to limit the indications of storage conditions to those that are achievable in practice. The basis for choosing storage conditions should be stability study results ».
Table - Generalized conditions for conducting stability tests according to ICH Q1
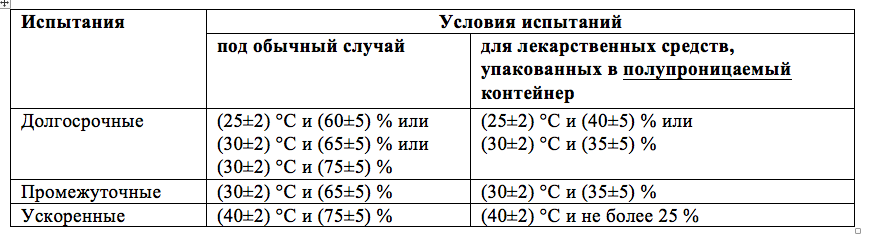
Thus, we come to the understanding that in determining the permissible range of humidity, it is necessary to proceed from the requirements stated in ICH Q1A. The document gives clear guidance on the values \u200b\u200bof relative humidity during the study (see table). It is obvious that the standardized value of relative humidity is in the range of 40-60% for an ambient temperature of 25 ° C, and in the range of 35-65% for the temperature range of +30 ° C, with permissible short-term fluctuations within ± 5%.
Hence the conclusion: during storage, it is necessary to ensure a relative humidity of up to 60-65%. These are the so-called the main storage conditions. Note that it is correct to normalize the value of relative humidity in the form of a one-sided interval without a lower boundary. Establishing a lower limit is at least not justified by the fact that if the quality of the drug worsens at low humidity, this is not a drug shortage, but a direct result of the use of poor quality packaging or its improper choice for HFRS (see ICH Q1A).
An alternative to this statement, obviously, can be pharmacopoeial requirements. The United States Pharmacopeia (USP) gives a clear definition of a dry place as “ a place with a relative humidity of not more than 40% at room temperatureor equivalent vapor pressure at a different temperature". Until recently, a similar definition was proposed by the Russian Pharmacopoeia GF XII ed. In the current, thirteenth, its edition, a dry place already refers to an environment with a relative humidity of not more than 50% (GF XIII, vol. 1, p. 213): “ Medicines which, in contact with water, moisture, can emit gases and the like, are moisture sensitive. Marking moisture sensitive Drugs, usually, contains an indication: “Store in a dry place”. When storing such drugs, it is necessary to create conditionsso that the relative humidity does not exceed 50% at room temperature (under normal storage conditions) or the equivalent vapor pressure at a different temperature". Other regulatory documents in the field of drug circulation do not standardize relative humidity.
However, if the labeling of a medicinal product (substance, finished product) contains the phrase “Store in a dry place”, it turns out that: a) it refers to substances whose degradation is critically dependent on excess moisture and b) its packaging does not provide reliable protection against moisture (based on from stability data), which means that during storage it is necessary to maintain relative humidity at a level of up to 40-50%, depending on national pharmacopoeial requirements.
We will separately consider the situation when a drug has a chance of changing the package (for example, storing tablets or capsules in organizers for dosed administration by the consumer) or opening it repeatedly (for example, storing tablets or capsules in a multi-dose container), and the drug is considered moisture-sensitive, then its warning label should be marked “Store in a tightly sealed container” or “Store in its original packaging”, which cannot be considered an analogue of “Store in a dry place ". Accordingly, when storing such preparations, it is sufficient to adhere to the basic storage conditions (up to 60% relative humidity).
It is important to pay attention to the following clarification. It may turn out that the intermediate product and (or) bulk product (for example, tablet mass, tablets) require storage in a dry place (up to 40% relative humidity), and for a product packaged in primary packaging with good moisture-proof properties (for example, contour cell-free packaging made of aluminum foil) basic storage conditions will be considered acceptable - relative humidity up to 60-65%, depending on the declared temperature regime.
"Secrets" of sealed packaging
The American Pharmacopoeia (USP) has another beautiful and very useful phrase: “ storage in any sealed packaging is considered dry storage". Good phrase. An equally attractive phrase is found in ICH Q1 guidelines, clause 2.2.7.2: “ Moisture sensitivity is not a problem for drugs packaged in airtight containers that provide constantmoisture barrier. Respectively, for preparations in sealed containersstability studies can be conducted in any controlled environment or environmental humidity "(Ie, in any uncontrolled conditions).
Returning to the Russian Pharmacopoeia (State Pharmacopoeia, XIIIth ed., Volume 1, p. 213) - there is also a similar phrase: “ ... Fulfillment of the requirement<хранить в сухом месте> as an alternative, provides for the storage of a moisture-sensitive drug in an airtight (moisture-proof) consumer packaging that provides the specified protection and storage conditions when handling the drug". Floridly does not quite fit the definition of “airtight container,” but essentially the same.
An interesting conclusion immediately suggests itself. If the drug (APS, drug) is in an airtight container, then this automatically enforces the requirement"Store in a dry place." We are instructed to store the drug in a dry place - this is how we store<in a sealed container\u003e. It is not even necessary to confirm the acceptability of the protective functions of the package (in terms of humidity) when studying stability, if its tightness is proved (shown).
And if at the same time during the production the tightness of the container is confirmed, then we can generally refuse to control the storage conditions by humidity. According to the definition given in the European Pharmacopoeia (3.2), a container obtained by fusing a homogeneous material (for example, glass ampoules) is called hermetic. Also, the tightness can be confirmed by checking at the stages of manufacture and (or) validation of the capping process (for example, for bottles with corking elements, see Appendix 1 GMP, 61, 117, 123) and even for blisters at the stages of production control.
Humidity in refrigerators and freezers
There are no normative guidelines or requirements for maintaining one or another value of relative humidity in refrigerators and freezers. Also, the ICH Q1A guidelines, pharmacopoeial monographs do not standardize humidity for stability studies in refrigerators and freezers.
This is due to the fact that in these storage areas the amount of moisture is much less than in air at a temperature of + 15 + 25 ° C. Based on the data of Fig. 2 it is obvious that the air in the refrigerator (+5 о С) even at 100% relative humidity contains moisture almost 1.5 times less than the air of the warehouse zone +20 о С at a humidity of 50%. And in the freezer, this ratio is already 8: 1. In addition, it so happened that most medicines that require low-temperature storage have sealed packaging, which means that by definition they are stored in a dry place.
At the same time, the cooling of warm air entering the refrigerator or freezer (for example, through cracks, when opening doors, etc.) will inevitably provoke condensation of excess moisture (up to 4-5 g / m 3) inside the chamber and, at certain conditions, can lead to moisture entering the group and (or) transport packaging, the formation of puddles, icing of surfaces and even the development of fungus and mold.
Only any value of relative humidity does not save from this. The issue of removal of excess moisture should be addressed at the design stage of low-temperature chambers and (or) zones and confirmed by the relevant qualification results.
Conclusion
The term “dry place”, which is used in various regulatory documents (GMP, GDP, Pharmacopoeia), and also applied to the labeling of drugs, has different meanings, and depends not only on regional standards, but also on the sensitivity of products to moisture and the properties of containers used for packaging. Relative humidity at a level of up to 60% in most cases provides favorable storage conditions for most drugs in pharmaceutical warehouses, meets pharmacopoeial requirements, and falls under the definition of “dry place”, since it does not exceed the values \u200b\u200bfor which it is customary to use the term “high humidity”, "dampness".
However, if the product is sensitive to moisture and packaged in containers that cannot protect it from moisture, it is required special storage conditions - in a place where relative humidity does not exceed 40%. Or vice versa, if low humidity can cause containers to lose their protective properties (for example, drying of silicone or rubber capping elements), and (or) quality indicators of the dosage form (for example, cracking of the shell of hard gelatin capsules or the shell of tablets, loss (evaporation) of the solvent from alcohol and aqueous solutions) - it is necessary to ensure storage of products at a relative humidity of at least 25-30%.
Manufacturers and distributors should always pay attention to warning labels on the labels affixed to the packaging of AFS, FPP, and other products. The presence of the inscriptions "Store in a dry place" or vice versa, "Store at a moisture content of at least 35%" - require maintenance special storage conditions depending on the norms established in the national pharmacopeia.

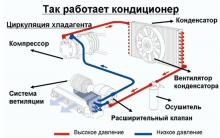
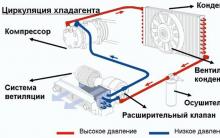






The best material for car trim
Principles of hardening the body
Do-it-yourself compressor - with minimal scrap costs
Which is better: do-it-yourself or factory-made compressor for painting a car
Causes of fuel pump malfunctions